View information documentation regarding 2-Butanol including CAS MSDS more. Mn VII is reduced under acidic conditions to Mn IV or Mn II according to the half-reaction s shown below with the indicated cell potentials 1.

CH 3 CH OHCH 2 CH 3 CH 3 C OCH 2 CH 3 H 2 This is used to produce approximately 700 million kilograms yearly.
2 butanol oxidation product. When H2O2 acts as an oxidizing agent the H2O2 must be reduced. Therefore the product from it will be water in which oxygen hasan oxidation number of -2 whereas in H2O2 has an oxidation. Dichromate ion is a strong oxidizing agent thus oxidation of 2-butanol secondary alcohol will give 2-butanone a ketone 1K views There was an error loading more items.
What is the product of oxidation of butanal. Peroxygenase for the hydroxylation of the inert gaseous substrate butane to 2-butanol in a bubble column reactor. Oxidation of butan-2-ol 2-butanol by acidified dichromate solution produces butan-2-one 2-butanone or butanone as represented in the equation below.
Decrease in conversion and yield may be due to complete oxidation of 2-butanol to undesired products like CO x methanol acetone and water at higher temperature. An appropriate and suitable reaction condition was always required for partial oxidation. What is the product of the oxidation of 2-butanol.
Methyl propyl ketone butane ethyl methyl ketone 2-butanal. Butanol is oxidised by sodium dichromate Na 2 Cr 2 O 7 acidified in dilute sulphuric acid to form the aldehyde butanal. The oxidation of the alcohol to an aldehyde is indicated by the colour change of the dichromate solution as it is reduced from the orange colour of Cr 2.
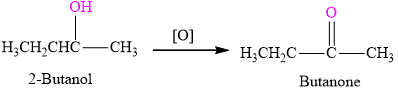
Sigma-Aldrich offers a number of 2-Butanol products. View information documentation regarding 2-Butanol including CAS MSDS more. Oxidation of 2-Butanol to 2-Butanone Shuowen Chen CHEM-2380-B1 Oct 27 2015 Introduction The purpose of this experiment was to oxidize 2-butanol to 2-butanone using chromic acid and prepare a 2 4-dintropenylhydrazone in order to characterize the 2-butanone.
Following the reaction IR and NMR spectra would also be used to indicate the purification of the liquid 2-butanone. The half-reaction and oxidation potential. Mn VII is reduced under acidic conditions to Mn IV or Mn II according to the half-reaction s shown below with the indicated cell potentials 1.
1 M n O 4 4 H 3 e M n O 2 2 H 2 O E o 168 V. 2 M n O 4 8 H 5 e M n 2 4 H 2. What Is The Oxidation Product For 3-methyl-2-butanol.
This problem has been solved. What is the oxidation product for 3-methyl-2-butanol. Expert Answer 100 1 rating Previous question Next question.
MA catalyst conferred a maximum 2-butanol conversion of 51 and 88 selectivity towards MEK. XPS analysis revealed that Mn in MA catalyst exists in 2 and 3 oxidation states and responsible for 2-butanol oxidation. Moreover it was found that the acidity of.
This resulted in the production of 95mM 2butanol and 09mM butanone the overoxidation product of 2butanol over the course of 6h. The measured TTN in this experiment was approximately 6500. Jones oxidation of 2-butanol - Free download as Word Doc doc docx PDF File pdf Text File txt or read online for free.
A simple reaction with chromium VI to create a ketone. Butanone may be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol using a catalyst is catalyzed by copper zinc or bronze.
CH 3 CH OHCH 2 CH 3 CH 3 C OCH 2 CH 3 H 2 This is used to produce approximately 700 million kilograms yearly.
