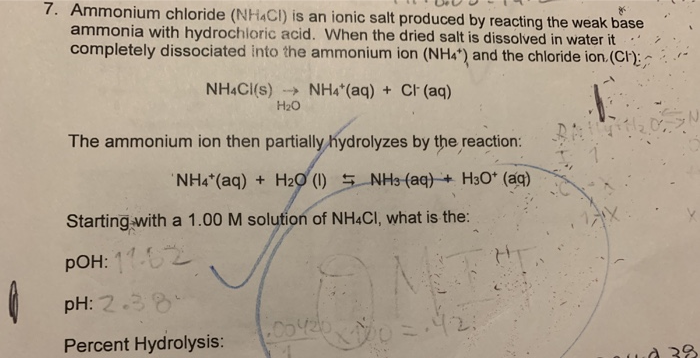
Classify the following solutions as acids bases or salts ammonium hydroxide barium chloride sodium chloride sodium hydroxide H2SO4 and HNO3. Didecyl dimethyl ammonium chloride is a clear yellow liquid yellowish powder or colorless crystals.
Ammonium chloride sodium hydroxide will form precipitate with ammonia gas.
Ammonium chloride acid or base. So Is NH4Cl an acid or base. NH4Cl is acidic salt in nature formed from neutralization of strong acid HCl and weak base NH4OH. The aqueous solution of ammonium chloride is slightly acidic having pH value range from 45 to 6.
Ammonium chloride is a strong electrolyte because it dissolved fully into ions or 100 ionized in aqueous solution. Ammonium chloride is used as a systemic acidifier in patients with metabolic alkalosis resulting from chloride loss following vomiting gastric suction gastric fistula drainage and pyloric stenosis. Ammonium chloride also has been used in the treatment of diuretic-induced chloride depletion.
The chloride is not a base and the ammonium is a weak acid. So we can say that the ammonium chloride acts as a weak acid. But since it dissolves fully into.
As we all know ammonium chloride is a acid. And what explains this is its salt hydrolysis. It breaks down into ammonium and chloride ions.
Ammonium react with water to produce hydronium or hydrogen ions. According to Bronsted-Lowrys definition the conjugate base of HCl would be HCl without a proton which a chloride ion. NH4Cl is an acidic salt that would produce HCl and ammonium in water.
Classify the following solutions as acids bases or salts ammonium hydroxide barium chloride sodium chloride sodium hydroxide H2SO4 and HNO3. In chemistry neutralization or neutralisation see spelling differences is a chemical reaction in which an acid and a base react quantitatively with each other. In ammonium cation and ammonia oxidation number of nitrogen atom is -3.
What will be the ammonium hydroxide and NaCl reaction. Ammonium hydroxide NH 4 OH is a weak base and NaCl is a salt. There is no releasing of ammonia gas when ammonium hydroxide is mixed with NaCl.
Ammonium chloride sodium hydroxide will form precipitate with ammonia gas. Ammonium chloride is a neutral salt but its solution is slightly acidic because ammonium ion N H 4 is a strong conjugate acid of a weak base ammonia and chloride ion C l is weak conjugate base of strong acid H C lHence in the solution the conjugate acid N H 4. Didecyl dimethyl ammonium chloride is a clear yellow liquid yellowish powder or colorless crystals.
It has a mushroom-like odor. It is moderately soluble in water. Didecyl dimethyl ammonium chloride is used as an antimicrobial.
Aluminum chloride AlCl3 is lewis acid What is an acid base neutral. Name the acid and base that would be used to prepare the following salts. I Potassium sulphate ii Ammonium chloride.
Asked Jul 10 2018 in Chemistry by Anukriti bharti 381k points acids bases and salts. Ammonium Chloride Theory In this titration aqueous solution of ammonium chloride is first treated with formaldehyde which results in the liberation of hydrochloric acid equivalent to the amount of ammonium chloride present in the solution. This hydrochloric in turn reacts with sodium hydroxide.
Chemical equation 4NH4Cl 4H 2O 4NH 4OH 4HCl Ammonium. Blood and urine samples were collected monthly to evaluate acid-base parameters plasma parathyroid hormone PTH and 125-dihydroxycholecalciferol levels. Ammonium chloride-treated cats had significantly lower blood and urinary pH and lower blood bicarbonate concentrations.
TMAH is a very strong base. One of the industrial uses of TMAH is for the anisotropic etching of silicon. It is used as a basic solvent in the development of acidic photoresist in the photolithography process and is highly effective in stripping photoresist.
If systemic acidosis is not present the acidifying agent ammonium chloride can be given orally in a dose of 01 gkg of body weight daily for 3 to 5 days or as a single dose of the same cumulative amount 121Urine is then collected hourly from 2 to 8 hours. In our experience the 3-day test gives more reliable results and is preferable as it allows time for a maximal increase in NH 4. Ammonium Chloride HCl NH3 NH4Cl Hydrochloric acid ammonia hydroxide.
Similarly if a base for example sodium hydroxide NaOH is added it will react with the acid in the buffer NH 4. NH 4 OH-NH 3 H 2 O. This is how a buffer maintains a near constant pH.
Every buffer is made up of a conjugate acid-base pair. If an acid is added to the buffer it is neutralized by the base. If a base is added to the.
