
Steam is the ultimate destination for playing discussing and creating games. 22064 MPa Critical Temperature 37395C.
The steam must be reheated or superheated in order to avoid damages that could be caused to blades of steam turbine by low quality steam.
Cp of superheated steam. Specific Heat of Superheated Steam. Viscosity of Superheated Steam. MPa s Pa s cP kgf sm² lbf sin².
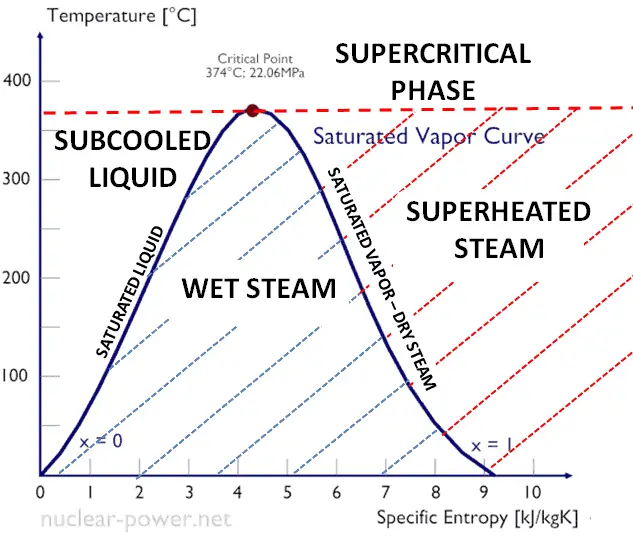
The superheated steam region depicts steam at a temperature higher than its saturation temperature. Should saturated steam be heated at constant pressure its temperature will rise producing superheated steam. Set your preferences for these steam tables.
- You cannot use commas as decimal points. Please use periods. Specific Enthalpy of Super Heated Steam can be calculated from the regular equation hs hg Cp Ts - Tf Cp - Specific Heat of Steam at Constant Pressure which can be considered as 1860 KJKgC Ts - Temperature of super heated Steam T f - Saturation temperature ie 100C.
TurestillthewellknownvaluehederivedCp048hasbeen acceptedgenerally forall temperatures and pressures. Laterdeterminationswhich have been made show thatCp varies. H superheated h sat.
Vapor T_sat for given boiler P Cp T_steam - T_sat The issue came up when evaluating specific heat constant for superheated steam. I used Shomates Equation. Cp A Bt Ct2 Dt3 Et2.
You may use this calculator to find Cp of Saturated Superheated stem. Online calculation of properties of water and steam For normal calculations Saturated Steam at Atmospheric pressure you may take value of Cp for steam as 2 kJkgK. Compressed Water and Superheated Steam 001 MPa t s 45806 C 002 MPa t s 60058 C 003 MPa t s 69095 C v ρh s t Cv h s v ρ h s 1010 27 98983 19181 0649 20 t sL 1017 16 98313 25142 0832 02 t sL 1022 24 97825 28927 0944 07 14 670.
0068 166 25839 81488 t. Vacuum steam is the general term used for saturated steam at temperatures below 100C. Example - Boiling Water at 100 o C 0 bar 100 kPa Atmospheric Pressure.
At atmospheric pressure 0 bar g absolute 1 bar water boils at 100 o C and 41751 kJ of energy is required to heat 1 kg of water from 0 o C to evaporating temperature 100 o C. 22064 MPa Critical Temperature 37395C. Ideal Gas Constant of Steam.
R 04615 kJkgK. Specific Heat Capacity of liquid water. C H2O 418 kJkgC.
Specific heat C is the amount of heat required to change the temperature of a mass unit of a substance by one degree. Isobaric specific heat Cp is used for substances in a constant pressure ΔP 0 system. Isochoric specific heat Cv is used for substances in a constant-volume.
Steam is the ultimate destination for playing discussing and creating games. Properties of Superheated Steam specific volume cubic feet per pound h g total heat of steam BTU per pound Pressure Lbs. Total Temperature –Degrees Fahrenheit t Abs.
P Gage P Sat. Temp t 350 400 500 600 700 800 900 1000 1100 1300 1500 150 03 2130 3 h g 3193 9 1216. 2 3396 3 1239.
9 3798 5 1287. The steam must be reheated or superheated in order to avoid damages that could be caused to blades of steam turbine by low quality steam. High content of water droplets can cause the rapid impingement and erosion of the blades which occurs when condensed water is blasted onto the blades.
To prevent this condensate drains are installed in the steam piping leading to the turbine. The reheater heats the steam point D and then the steam. Where to find Cp values for steam for different pressures at different temperatures.
I can not find a table for water vapour for Specific heat at const. Pressure values for at varying temperatures. Callendarholds thatatzero pressure C should havea constant value but Knoblauchand Jakob fromtheirdirectdetermin-.
Blue - viscosity of water 1 Supercritical fluids are phases of substances at high pressures above their critical temperatures. Supercritical water is superheated steam under high pressure of more than 32062 psia 200 atm. Under these conditions supercritical water decomposes to many substances.