
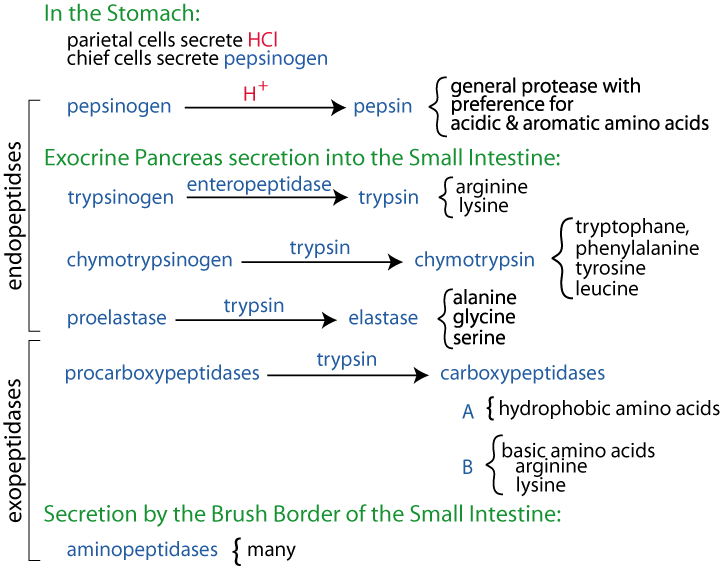
Once in the intestine an enzyme called enteropeptidase which is secreted from intestinal cells cuts off a small piece of trypsinogen to produce the active trypsin enzyme. Not normally used for protein IDs more for PTM analysis.
Fragmentation analysis of the intact protein Protein Sequencing Top down.
Does trypsin digest proteins. Annonce Expertise on Every Level to Craft Science Technology Solutions in Life Science. For research or for further manufacturing use Available from Sigma-Aldrich. The trypsin digestion can be stopped by freezing or by lowering the pH of the reaction below pH 4 by adding formic acetic or trifluoroacetic acid trypsin will regain activity when the pH is raised above pH 4.
Digested samples can be stored at -20C. Correspondingly do proteases digest. Trypsin is one of the enzymes used to digest proteins.
It breaks the peptide bonds at the C terminal of the basic amino acids lysine and arginine. Trypsin is very similar to another protein. Trypsin is an enzyme that helps us digest protein.
In the small intestine trypsin breaks down proteins continuing the process of digestion that began in the stomach. It may also be referred to as a proteolytic enzyme or proteinase. Trypsin is a protein-digesting enzyme present in pancreatic juices secreted into your small intestine during a meal.
Your pancreas secretes trypsin as an inactive proenzyme called trypsinogen. Once in the intestine an enzyme called enteropeptidase which is secreted from intestinal cells cuts off a small piece of trypsinogen to produce the active trypsin enzyme. Activated trypsin in turn helps break down food proteins.
Trypsin has become the gold standard for protein digestion to peptides for shotgun proteomics. Trypsin is a serine protease. It cleaves proteins into peptides with an average size of 700-1500 daltons which is in the ideal range for MS Laskay et al 2013.
It is highly specific cutting at the carboxyl side of arginine and lysine residues. Digested in serum for 15 min 30 min 1 h 3 h 6 h and 16 h overnight with porcine trypsin at 37 C. The protein to trypsin ratio was 130.
Digestion efficiency was calculated from the normalized averaged peak areas n4 obtained from the MRM chromatograms. Trypsin immobilisation In contrast to conventoi nal procedures whci h digest proteins using free trypsin in solution the SMART Dgi est kit is based on immobzilied trypsin. This has a number of advantages.
Tethering trypsin to a solid substrate allows it. Digest the protein with a specific protease most often trypsin for Peptide Mass Fingerprinting. Use proteolysis CID and peptide fragmentation to identify sections of protein sequence.
Fragmentation analysis of the intact protein Protein Sequencing Top down. Not normally used for protein IDs more for PTM analysis. Trypsin is an enzyme that helps us digest protein.
In the small intestine trypsin breaks down proteins continuing the process of digestion that began in the stomach. It may also be referred to as. As previously stated trypsin is released into the small intestine in order to further digestion of large protein molecules.
But addition to trypsin an activator of many proteases other enzymes such as chymotrypsin aids in the digestion of proteins. Though trypsin did appear to have done some digestion within the test tube it still wasnt capable of digesting protein on its own. Trypsin is a digestive enzyme that breaks down proteins in the digestive system of many vertebrates.
It is found in the pancreatic juice. Trypsin cleaves lysine and arginine residues of the carboxy-terminal of peptides. However it does not cleave.
Annonce Expertise on Every Level to Craft Science Technology Solutions in Life Science. For research or for further manufacturing use Available from Sigma-Aldrich.