Physical Equilibrium Part II. Iodine and carbon tetrachloride are both nonpolar so the liquid dissolves the solid.

Chemistry Kinetic Molecular Theory.
Like dissolves like chemistry. Like dissolves like is an expression used by chemists to remember how some solvents work. It refers to polar and nonpolar solvents and solutes. Oil is non polar.
Water will not dissolve oil. Salt NaCl is ionic which is considered extremely polar. Iodine and carbon tetrachloride are both nonpolar so the liquid dissolves the solid.
The water layer does not dissolve the iodine because water is polar. A similar explanation can be used to show why the permanganate dissolves in the water and not the tetrachloride but it should be sufficient to say that like dissolves like. Like Dissolves Like OpenChem General Chemistry 1B.
Physical Equilibrium Part II. If playback doesnt begin shortly try restarting your device. Videos you watch may be added to the TVs watch history and influence TV recommendations.
To avoid this cancel and sign in to YouTube on your computer. An error occurred while retrieving. Chemists say that like dissolves like meaning that substances with similar chemical characteristics will dissolve in each other.
Specifically polar solvents tend to dissolve polar solutes and non-polar solvents tend to dissolve non-polar solutes while non-polar and polar substances are Immiscible do not mix. This paper describes a demonstration and a straightforward experiment using the guided-inquiry approach for the like dissolves like principle that is implemented at the beginning of the academic year. This experiment extends the students understanding of the concept of solubility of organic molecules and its relationship to chemical structure.
The predicted miscibility shows remarkable agreement with known data thus providing a quantitative basis for the empirical like-dissolves-like rule. Liquid mixtures are ubiquitous. Polar solutes dissolve in polar solvents.
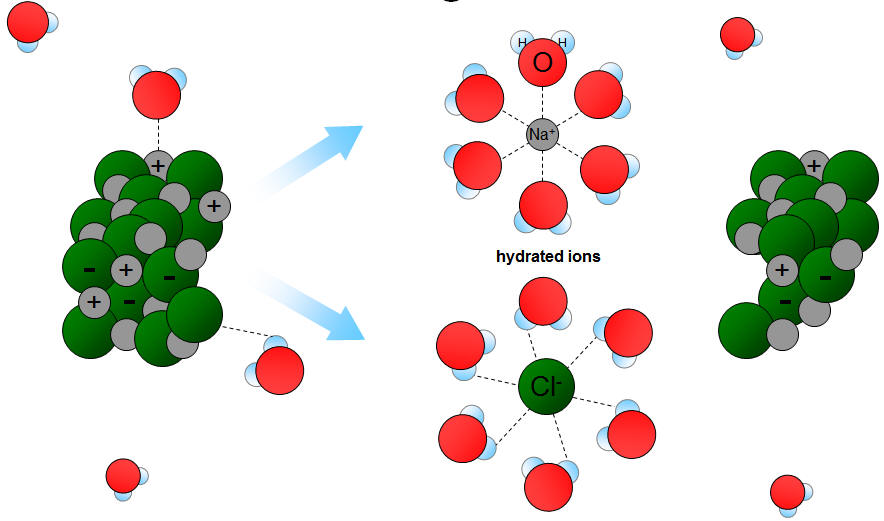
Like dissolves like means if any substance is polar then polar and ionic substances will be dissolved in it. If the solvent is non-polar then the non-polar substances only can be dissolved in it. In chemistry the like dissolves like rule refers to solubility and polar and non-polar substances.
Solute means the substance that is dissolved into a larger substance the solvent. But in more accurate terms. The solute in a solut.
A short video demonstrating that a polar solvent water will dissolve an ionic solute but not a nonpolar solute and that a nonpolar solute hexane will di. Methanol and ethanol in particular are infinitely miscible with water. On the other hand non-polar solvents such as hexanes are capable of sometimes dissolving non-polar solutes.
Hexanes is miscible with ethanol because of the two-carbon chain but IMMISCIBLE with methanol a. LIKE DISSOLVES LIKE Kathryn M. Wagner Princeton University Chemistry Department kmwagnerprincetonedu Brief description of demonstration Three clear liquids form three distinct layers in a cylinder.
Iodine crystals sprinkled on the top layer sink and form pink solutions with the top and bottom layers but do not dissolve in the middle. There is a commonly used principle for predicting the miscibility of molecular liquids in each other. More specific statements of this generalization are.
Substances whose molecules are polar are miscible with other substances whose molecules are polar. Like dissolves like is a saying in the world of chemistry and means that polar solvents are best at dissolving polar substances and non-polar solvents are best at dissolving non-polar substances. Solubility Rule Like Dissolves Like Purpose.
Students will experience the affect a substances composition and structure has on its solubility. Distilled water food coloring apron corn starch vegetable oil Canola oil beakers beaker tongs glucose hot. The chemistry principal of like dissolves like explains that polar substances will dissolve in each other.
Similarly a covalent will dissolve another covalent. CHEMISTRY Like dissolves like. A first-principles theory for predicting liquid miscibility and mixture dielectric constant Bilin Zhuang123 Gabriele Ramanauskaite2 Zhao Yuan Koa2 Zhen-Gang Wang1 Liquid mixtures are ubiquitous.
Miscibility and dielectric constant are fundamental properties that govern the applications of liquid mixtures. Furthermore there is a general like dissolves like principle that explains that different solvents only extract phytochemicalssecondary metabolites that share a similar polarity. Only one rule to know here.
Like polarity dissolves like polarity. Watch more of this topic httpcltchus1FcyyF3GET MORE CLUTCHVISIT our website for mo. Demo 2 Like Dissolves Like WaterBased vs.
Permanent Markers Useful in Units. Solutions Physical Chemistry Organic Bonding Background and Uses This is about as easy a demo to do as there is and it has good Real World Connections. Chemistry Kinetic Molecular Theory.
Interaction of Matter Like Dissolves Like. View Like Dissolves Like in Google Docs. Friday September 19 2014 255 PM.