
An isocyanate excess is generally not used. Catalyse the isocyanate-hydroxyl reaction by complex formation with both isocyanate and hydroxyl groups.
In a study involving various.
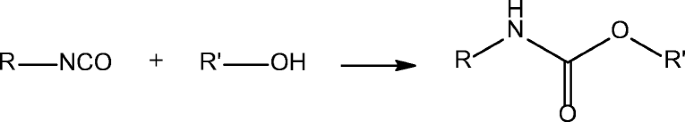
Reaction of isocyanate with alcohol. Tonate catalyzed reaction between a-naphthyl isocyanate and n-butyl alcohol is shown in Fig. The system most exhaustively studied was the reaction of a-naphthyl isocyanate with n-butyl alcohol using ferric acetylacetonate as catalyst and diethylene glycol diethyl ether as solvent. Initial slopes for the reaction at several.
The alcoholysis of isocyanate was examined experimentally for 2-propanol and cyclohexanol in low and high concentrations. It is suggested that either two or three molecules of the alcohol are implicated from the kinetic study while the reaction with trimers becomes dominant at high alcohol concentrations. In accordance with these results theoretical study suggests an active.
N Reaction involving a very reactive chemical group N C O with an alcohol to form a highly crosslinked polymer importance in polyurethane formation. C Principles of Polymerization Wiley New York 2004. New insight into the kinetics of diisocyanate-alcohol reactions by high-performance liquid chromatography and mass spectrometry.
Journal of Applied Polymer Science 2015 132 25 na-na. The kinetics of the reaction between 26toluene diisocyanate 26TDI and alcohols with varying carbon chain lengths and a primary or secondary hydroxyl group in the absence and presence of tinII 2ethylhexanoate TEH as a catalyst were studied. The alcohols were applied in high molar excess relative to 26TDI to obtain a pseudofirstorder dependence on 26TDI.
Based on the kinetic results it was found that i 26TDI reacts faster with alcohols. Isocyanates especially diisocyanates and polyisocyanates are important monomers that undergo a broad range of chemical reactions. For example they can react with compounds containing active hydrogen atoms like amines alcohols mercaptanes water and carboxylic acids or they can react with themselves to form dimers.
This study is concerned with some kinetic features of the reaction of nbutyl isocyanate with polyvinyl alcohol without any catalyst and using triethylene diamine as catalyst. The structure of the resulting polymers was determined by means of IR 1 H and 13 CNMR spectroscopy as well as by chemical analysis. Vinyl alcoholvinyl butyl urethane VALVBU copolymers were obtained.
It has been found a. The objective of this work was to obtain primary amine groups on the surface of polyvinyl alcohol films by means of a reaction with hexamethylene diisocyanate. The reaction was run in such a way as to minimize the internal crosslinking by employing a large excess of hexamethylene diisocyanate in toluene and then hydrolyzing the unreacted isocyanate endgroup to primary amine.
Which promotes the isocyanate alcohol reaction in contras t to. The isocyanate water r eaction. In a study involving various.
Di ff erent metal based catalysts Edelmann concluded. An isocyanate excess is generally not used. There is another field in which the reaction of isocyanates in excess with trace levels.
The polymerization reaction 1 between an alcohol and an isocyanate is exothermic and releases about 24 kcalmol of urethane. Isocyanates reactivity with alcohols is moderate Table 22 being usually catalyzed by bases mainly tertiary amines or organometals. Reactivity is influenced by structure and primary secondary and tertiary hydroxyls have decreasing reactivity due to neighboring methyl.
Catalyse the isocyanate-hydroxyl reaction by complex formation with both isocyanate and hydroxyl groups. The positive metal centre interacts with electron rich oxygen atom of both the isocyanate and hydroxyl groups forming an intermediate complex which by further rearrangement results in the formation of urethane bonds. Mono- and diphenols add to trimethyl isocyanate on heating to give the corresponding aryl urethanes.
Ethanol reacts with trimethylsilyl isocyanate to give ethyl urethane and ethyl allophanate in a ratio determined by reaction conditions. KINETIC EQUATIONS A number of equations have been suggested to describe the kinetics of the reaction of an isocyanate with an alcohol. In a classic series of papers Baker and co- workers4-6 studied the catalysed and uncatalysed re- action of phenyl isocyanate with simple alcohols.
Reactions of aliphatic isocyanates with a phenolic ester alcohol PHEA were investigated using 13C-NMR spectroscopy. PHEA has two reactive sites. A phenolic OH group and a secondary aliphatic OH.
This review focuses on catalysis the mechanism involved in the formation of Non-Isocyanate Polyurethanes NIPU from five-membered cyclic carbonates and the reaction.