Can be detected by absorbance 340 490 or 600nm Removed by filtration or centrifugation. Other practices that reduce the propensity for proteins to aggregate include.

We and others have shown that fusing aggregation-prone proteins to MBP can lead to the recovery of properly folded protein in many cases.
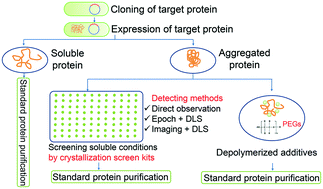
Soluble aggregates protein purification. We have developed a methodologically simple analytical procedure that qualitatively determines the aggregation ten-dency of a given protein. Because intriguingly protein deposi-tion correlates with the size of soluble aggregates we propose microdialysis as a routine screening method of both protein. Upon biophysical characterization of the protein by analytical ultracentrifugation and differential light scatteringit appears the protein exists as a solube aggregate and elutes off of a S200.
Extended purification procedures and the presence of soluble aggregates may accelerate protein insolubility. This nucleation process can be considerably avoided by early elimination of soluble aggregates SEC or other chromatographic procedures like ion exchange or mixed mode chromatography and a quick strategy of selection of a purification optimization procedure and. Im working on a 30 KDa His tagged protein that purifies well on Ni-NTA and comes up in the soluble fraction.
The protein however shows compromised activity and for long we hammered our heads against changing the assay conditions etc until we stumbled upon some gelfiltration and DLS results which show that almost 90 of the protein exists in the form of Soluble aggregatesCan someone tell me a good method of breaking these aggregates. To obtain the native ie correctly folded and hence active form of the protein from such aggregates four steps are usually followed. 1 the cells are lysed and the are aggregates 2 the cell wall and outer membrane components of the aggregates are removed 3 the aggregates are solubilized or extracted with strong protein denaturants and 4 the solubilized denatured proteins are folded with concomitant oxidation of reduced cysteine residues into the correct disulfide bonds.
Size exclusion chromatography SEC is used to separate soluble aggregates from mono-dispersed proteins in the void volume but again this procedure requires prior purification steps. Moreover SEC is unsuitable for large-scale industrial production due to the limited capacity of columns. Thus a simple and practical procedure to screen out soluble aggregates is required prior to downstream purification.
Quantitation of soluble aggregates in the cell culture is time-consuming and labor-intensive usually involving a purification step to remove the impurities that interfere with the subsequent size exclusion chromatography SEC analysis. If the ability of the protein to be concentrated is not known the concentration should proceed in incremental steps in order to avoid a change in the oligomeric state of the protein to soluble aggregates or insoluble aggregates. Take samples after each step to check protein concentration and oligomeric state of the protein by analytical gel filtration chromatography GF.
Soluble aggregates can be. We and others have shown that fusing aggregation-prone proteins to MBP can lead to the recovery of properly folded protein in many cases. A good example is TEV protease.
However it is certainly. In order to optimize the expression and purification of CEA N-A1 domains we evaluated bacteria cultivation conditions the length of N-A1 domains fusion systems GST- and MBP-tag IPTG concentrations and protein purification conditions. We have found that MBP-N-A1 fusion protein digested with TEV protease forms soluble aggregates composed of N-A1 domains and incompletely digested MBP-N-A1.
Protein aggregation is a complex process resulting in the formation of heterogeneous mixtures of aggregate populations that are closely linked. SOLUBLE RECOMBINANT PROTEINS Recombinant proteins that are not expressed in inclusion bodies either will be soluble inside the cell or if using an excretion vector will be extracellular or if E. Coli is the host possibly periplasmic.
They can be purified by conventional means. The glutathione S-transferase GST system is useful for increasing protein solubility and purifying soluble GST fusion proteins. However purifying half of the GST fusion proteins is still difficult because they are virtually insoluble under non-denaturing conditions.
To optimize a simple and rapid purification condition for GST-pyruvate kinase muscle 2 GST-PKM2 protein we used 1 sarkosyl for lysis and a 1200. Heat treatment can simplify the purification protocol of thermotolerant proteins as well as remove any soluble aggregate. Since the re-folding capability after heat-induced denaturation was previously correlated to higher performance during recombinant expression a unique heating step can be envisaged to screen constructs that can provide high yields of correctly-folded proteins.
A range of sizes and characteristics eg. Soluble or insoluble covalent or non-covalent reversible or irreversible. Protein aggregates span a broad size range from small oligomers nanometers to insoluble micron-sized aggregates that can contain millions of monomer units.
Aggregation needs to be carefully characterized and controlled during. Method for isolating and purifying soluble Aβ aggregates from human AD brain. Cortical tissue was dounce homogenized in sub-critical micelle concentration of the detergent CHAPS size forms of.
They inhibit protein aggregation by competing with proteins at hydrophobic surfaces. Polysorbate 20 and polysorbate 80 most common undergo auto-oxidation yielding reactive peroxides which cause degradation. Sugars protect proteins against dehydration by forming hydrogen bonds with the protein as water substitutes.
This increase thermal unfolding temperature and. Mechanisms of Protein Aggregation. 100μm 1mm or more.
Can be detected by eye 1 μm - 100μm. Can be detected by absorbance 340 490 or 600nm Removed by filtration or centrifugation. Soluble aggregates dimers-oligomers 10nm - 1 μm.
Buffer conditions that favour protein solubility at 4C may not necessarily prevent aggregation during freeze-thaw. Glycerol is often added to the protein sample as a cryoprotectant. Other practices that reduce the propensity for proteins to aggregate include.
Performing all purification steps at.