In the classic crystalline semiconductors electrons can have energies only within certain. Out of 5 valence electrons only 4 are involved in bond formation and the fifth electron is delocalized and can be easily provided to the conduction band.
While insulating materials may be.
Types of semiconductor in chemistry. In the classic crystalline semiconductors electrons can have energies only within certain. The name extrinsic semiconductor can be a bit misleading. While insulating materials may be.
Science Chemistry Solid State Semiconductors On the basis of electrical conductivity substances can be classified into three types conductors insulators and semiconductors. In this article we shall have a brief idea of semiconductors. N-Type semiconductors are negative charge carriers.
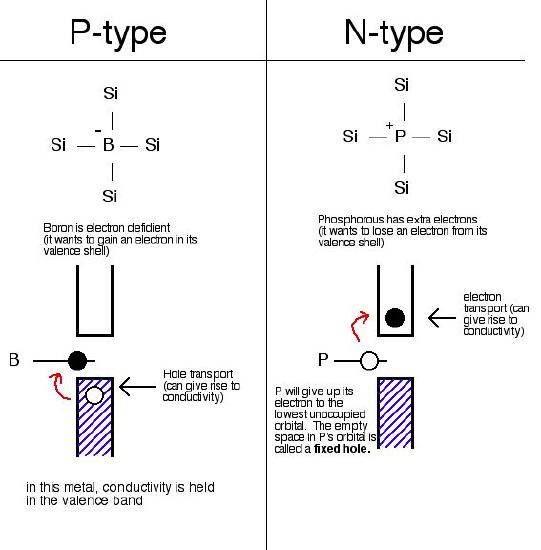
The impurity has more valence electrons than the host. P-Type semiconductors are positive charge carriers. The impurity has fewer valence electrons than the host.
99 rows Types of semiconductor materials. Group IV elemental semiconductors C Si Ge Sn. The two main types of semiconductors are.
I n -type semiconductor. Ii p -type semiconductor. The semiconductor whose increased conductivity is a result of negatively-charged electrons is called an n -type semiconductor.
When the crystal of a group 14 element such as Si or Ge is doped with a group 15 element such as P or. P-type semiconductors are materials doped with an element that has a lower valence state than the original material whereas an n-type material is one which has been doped with an element of a higher valence state. For reference we will consider silicon as that is one of the most widely used materials in these junctions.
The elemental semiconductors are those composed of single species of atoms such as silicon Si germanium Ge and tin Sn in column IV and selenium Se and tellurium Te in column VI of the periodic table. There are however numerous compound. The junctions which formed where n-type and p-type semiconductors are joined together is called pn junction.
A semiconductor diode is a device typically made up of a single p-n junction. The junction of a p-type and n-type semiconductor forms a depletion region where current conduction is reserved by the lack of mobile charge carriers. Semi conductors are the solids that have properties intermediate between metals insulatorsThey have only small difference in energy between the filled valence band empty conduction bandThe conductivity is also intermediate between that of a metal an insulator depends upon the number of electrons in the conduction band.
Semiconductors can be divided into two groups. Intrinsic semiconductors and extrinsic semiconductors. Extrinsic semiconductors are doped which means some small amounts of impurities are added to improve conductivity.
Intrinsic semiconductors are not dopedor more precisely they have the same number of electrons and holes. The solids with intermediate conductivities between insulators and conductors are termed semiconductors. I n- type semiconductor.
It is obtained by doping Si or Ge with a group 15 element like P. Out of 5 valence electrons only 4 are involved in bond formation and the fifth electron is delocalized and can be easily provided to the conduction band. There are two different kinds of semiconductors.
When voltage is applied to semiconductor devices electron current flows toward the positive side of the source and holes current flows towards the negative side of the source. Such a situation occurs only in a semiconductor material. Thus the holes are the majority carriers while electrons are the minority carriers in P-type materials.
Blue diamonds Type IIb which contain boron B impurities are an example of a naturally occurring P-type semiconductor. To a first approximation sufficiently doped P-type semiconductors can be thought of as only conducting holes.